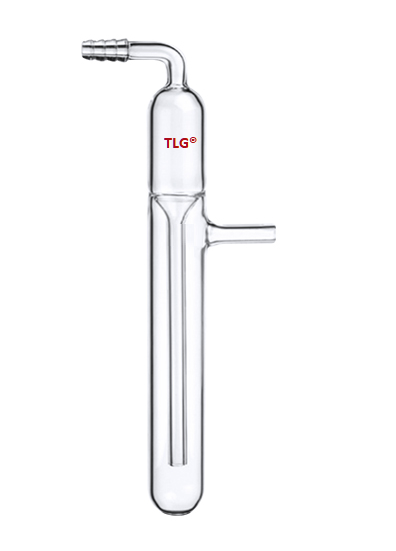
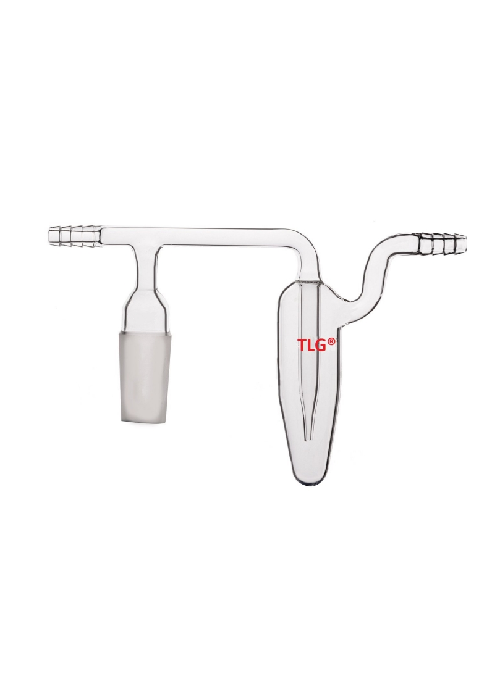
In chemistry a bubbler is a lab tool, usually a tiny glass bulb, that is used to keep a vacuum over a reaction vessel or to release reaction gases while keeping the reaction from becoming contaminated by air or moisture.
Since it is a one-way valve, gas can bubble out of a liquid (often oil or mercury) but air cannot bubble back in.
A more thorough description is provided below:
-
The Inert Atmosphere: In Schlenk lines or comparable systems, bubblers are frequently used to keep an inert atmosphere—typically nitrogen or argon—over a process, preventing oxidation or other interactions with ambient gases.
-
Pressure Relief: In the event that pressure builds up, they offer a means for gas to escape the reaction vessel, avoiding explosions or glassware damage.
-
One-way valve: When the gas flow is interrupted or the pressure is lowered, the liquid in the bubbler keeps air from getting into the reaction vessel.
-
Visual Indicator: By causing the gas to bubble through the liquid in the bubbler, the reaction conditions can be observed.
-
Liquids Used: Mercury bubblers are also used, however, mineral oil is frequently used because it is less hazardous and causes less splashing than mercury.

















 Filter
Filter