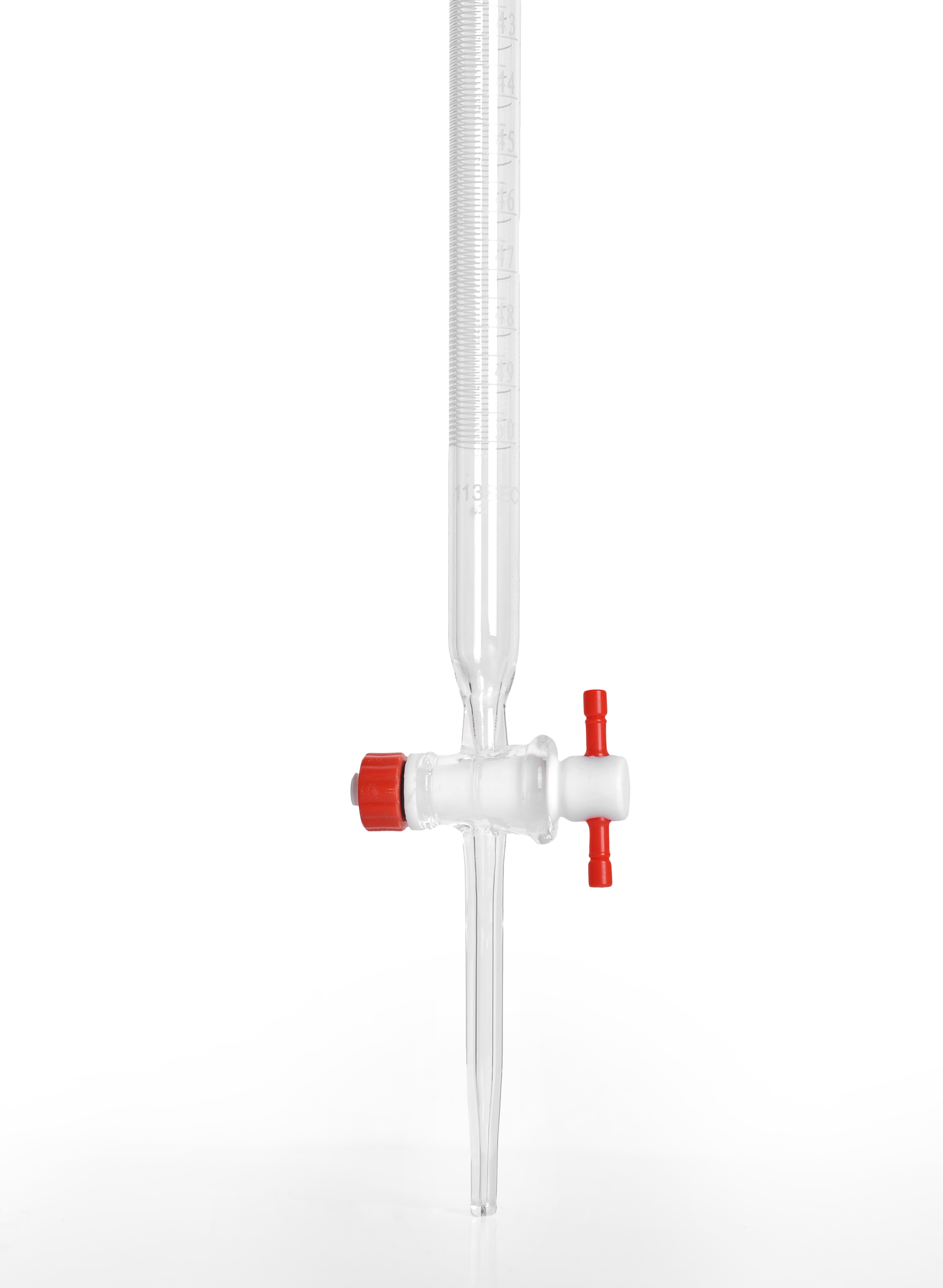
A separating funnel, often referred to as a separatory funnel or a decanting funnel, is mostly used to separate inert liquids according to changes in density. Its uses span from industrial procedures like water and oil separation to laboratory settings for liquid-liquid extractions.
Key applications of a separating funnel include:
-
Liquid-liquid extractions: These are often used in biology and chemistry to extract compounds from one liquid phase into another according to changes in solubility.
-
Immiscible liquid separation: The funnel makes it possible to carefully separate organic solvents from aqueous solutions or liquids that don't mix, like water and oil.
-
Industrial processes: Separation funnels are used in industries to separate diverse combinations, including oil and water, water and gasoline, and in the creation of cosmetics, medicines, and food goods.
-
Laboratory studies: Separating funnels are crucial for investigations involving liquid-liquid extractions, purifying chemicals, and evaluating solubility.
How a separating funnel works:
-
To the funnel are put two immiscible liquids.
-
The liquids are mixed by shaking the funnel.
-
After allowing the liquids to settle, the denser liquid forms the bottom layer.
-
The stopcock at the funnel's bottom allows the lower layer to be emptied.
-
You may either drain the top layer separately or keep it in the funnel.























 Filter
Filter