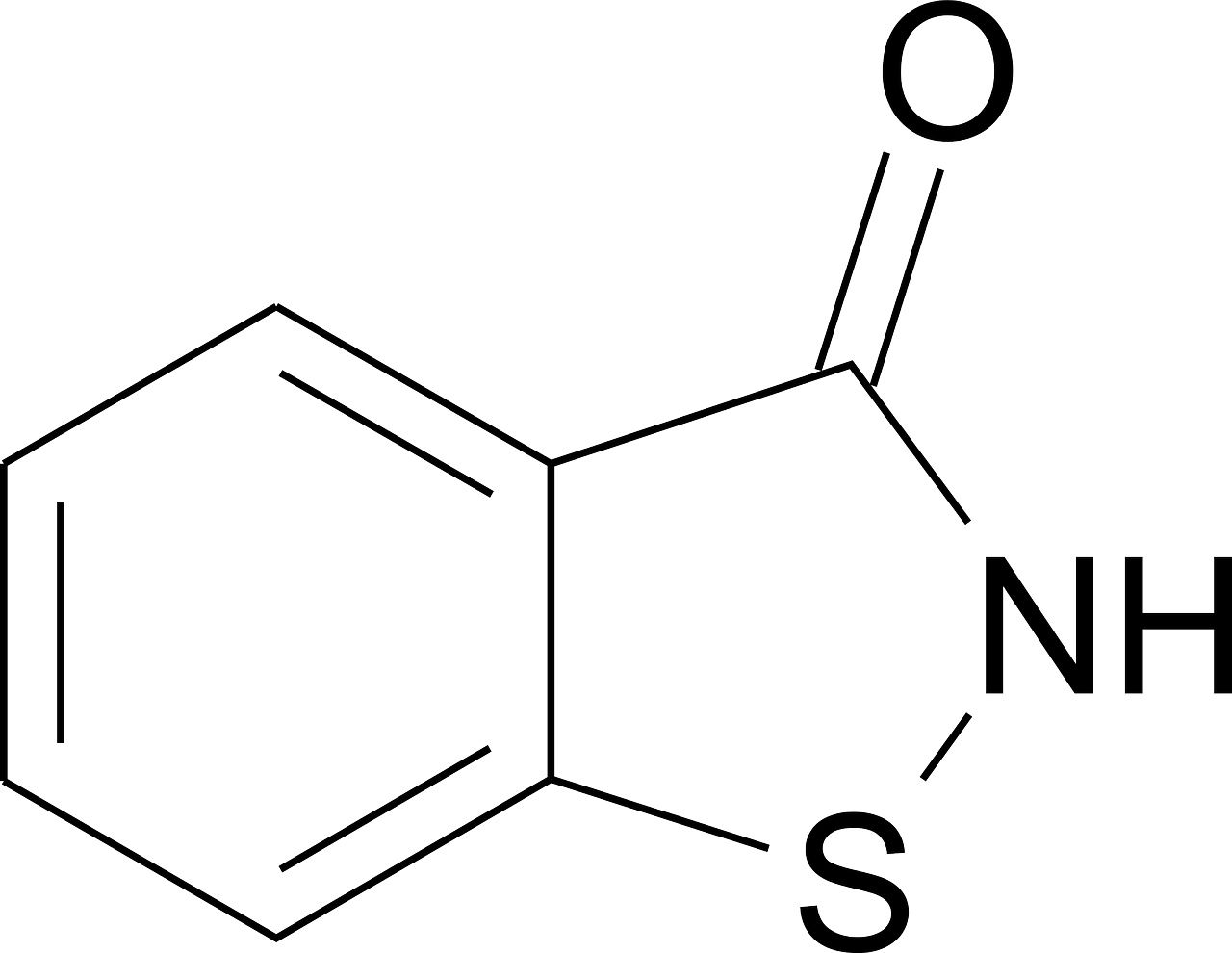
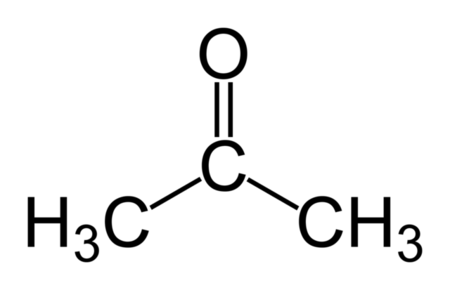
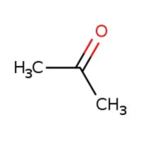
Acetone
CAS: 67-64-1 Molecular Formula: C3H6O Molecular Weight (g/mol): 58.08 MDL Number: MFCD00008765 InChI Key: CSCPPACGZOOCGX-UHFFFAOYSA-N Synonym: acetone,2-propanone,propanone,dimethyl ketone,methyl ketone,dimethylformaldehyde,pyroacetic ether,beta-ketopropane,dimethylketal,chevron acetone PubChem CID: 180 ChEBI: CHEBI:15347 IUPAC Name: propan-2-one SMILES: CC(C)=O


















 Filter
Filter